matter in our surroundings extra questions
CBSE Exam-Oriented | All Question Types | CBSE Class 9 Science
Looking to score high in Science? You’ve landed on the right page! Our Matter in Our Surroundings extra questions cover everything — from very short to long answer type questions, along with MCQs, assertion-reason, and case-based questions. Designed by experts and aligned with the latest CBSE pattern, these questions help you revise faster, understand concepts better, and write answers that fetch full marks.
👉 Start your smart prep now and conquer this chapter with confidence!
Matter In Our Surroundings Extra Questions (2025 Edition)
📄 Multiple Choice Questions (MCQs)
Q1. The property to flow is unique to fluids. Which statement is correct?
(A) Only gases behave like fluids.
(B) Gases and solids behave like fluids.
(C) Gases and liquids behave like fluids.
(D) Only liquids are fluids.
Answer: (C) is correct. Liquids and gases can flow easily due to weaker intermolecular forces.
(B) Gases and solids behave like fluids.
(C) Gases and liquids behave like fluids.
(D) Only liquids are fluids.
Answer: (C) is correct. Liquids and gases can flow easily due to weaker intermolecular forces.
Q2. Arrange the following in increasing order of force of attraction between particles: oxygen (gas), water (liquid), sugar (solid).
(A) Sugar < water < oxygen
(B) Oxygen < water < sugar
(C) Water < sugar < oxygen
(D) Water < oxygen < sugar
Answer: (B). Forces of attraction increase from gases → liquids → solids.
(B) Oxygen < water < sugar
(C) Water < sugar < oxygen
(D) Water < oxygen < sugar
Answer: (B). Forces of attraction increase from gases → liquids → solids.
Q3. Which of the following increases when temperature is raised?
(A) Diffusion
(B) Evaporation
(C) Expansion of gases
(D) All of the above
Answer: (D). Higher temperature increases kinetic energy, so all these processes increase.
(B) Evaporation
(C) Expansion of gases
(D) All of the above
Answer: (D). Higher temperature increases kinetic energy, so all these processes increase.
Q4. Which pair of conditions is most suitable for liquefying gases?
(A) Low pressure, low temperature
(B) High temperature, low pressure
(C) Low temperature, high pressure
(D) High temperature, high pressure
Answer: (C). High pressure brings particles closer, and low temperature removes kinetic energy.
(B) High temperature, low pressure
(C) Low temperature, high pressure
(D) High temperature, high pressure
Answer: (C). High pressure brings particles closer, and low temperature removes kinetic energy.
Q5. The SI unit of temperature is:
(A) Celsius
(B) Kelvin
(C) Fahrenheit
(D) Joule
Answer: (B) Kelvin
(B) Kelvin
(C) Fahrenheit
(D) Joule
Answer: (B) Kelvin
Q6. Choose the correct statement of the following:
(A) Conversion of solid into vapours without passing through the liquid state is called vaporisation
(B)Conversion of solid into vapour withoutpassing through the liquid state is called sublimation.
(C)Conversion of solid into liquid is called sublimation.
(D) Conversion of vapours into solid without passing through the liquid state is called freezing.
Answer: (B) : The conversion of liquid into gas(vapour) is called vaporisation. The conversion of liquid into solid is called freezing. The conversion of solid into liquid is called melting
(B)Conversion of solid into vapour withoutpassing through the liquid state is called sublimation.
(C)Conversion of solid into liquid is called sublimation.
(D) Conversion of vapours into solid without passing through the liquid state is called freezing.
Answer: (B) : The conversion of liquid into gas(vapour) is called vaporisation. The conversion of liquid into solid is called freezing. The conversion of solid into liquid is called melting
📄 Assertion and Reason Questions
Q1. Assertion (A): A gas exerts pressure on the walls of its container.
Reason (R): Gas particles move randomly and collide with the walls.
Reason (R): Gas particles move randomly and collide with the walls.
Answer: (A) Both A and R are true, and R is the correct explanation of A.
Explanation: The constant and random motion of gas particles causes them to collide with the container walls, exerting pressure.
Explanation: The constant and random motion of gas particles causes them to collide with the container walls, exerting pressure.
Q2. Assertion (A): Naphthalene disappears over time without leaving any residue.
Reason (R): It changes from solid to gas directly.
Reason (R): It changes from solid to gas directly.
Answer: (A) Both A and R are true, and R is the correct explanation of A.
Explanation: This process is called sublimation. Naphthalene sublimes into vapour, hence no liquid or residue is observed.
Explanation: This process is called sublimation. Naphthalene sublimes into vapour, hence no liquid or residue is observed.
Q3. Assertion (A): Ice at 0°C is more effective in cooling than water at 0°C.
Reason (R): Ice absorbs latent heat during melting.
Reason (R): Ice absorbs latent heat during melting.
Answer: (A) Both A and R are true, and R is the correct explanation of A.
Explanation: Ice uses latent heat of fusion to melt, taking more energy and thus providing better cooling.
Explanation: Ice uses latent heat of fusion to melt, taking more energy and thus providing better cooling.
Q4. Assertion (A): Gas molecules intermix (spread out) when released in air.
Reason (R): Diffusion is the spontaneous mixing of particles of two different substances.
Reason (R): Diffusion is the spontaneous mixing of particles of two different substances.
Answer: (B) Both A and R are true but R is not the correct explanation of A.
Explanation: Gas molecules intermix because they move randomly and fill space; diffusion is the general process that results (R), but A’s intermixing specifically occurs due to particle motion.
Explanation: Gas molecules intermix because they move randomly and fill space; diffusion is the general process that results (R), but A’s intermixing specifically occurs due to particle motion.
Q5. Assertion (A): A solid starts melting when heat is supplied.
Reason (R): The solid’s particles absorb the heat and break free from their fixed positions.
Reason (R): The solid’s particles absorb the heat and break free from their fixed positions.
Answer: (A) Both A and R are true, and R is the correct explanation of A.
Explanation: Supplying heat increases particle kinetic energy, weakening attractions and causing melting (fusion).
Explanation: Supplying heat increases particle kinetic energy, weakening attractions and causing melting (fusion).
📄 Very Short Answer Questions (1 Mark)
Q1. Why can't we see particles of matter with the naked eye?
Particles of matter are extremely small — so tiny that they cannot be seen without a microscope. Their size is in the nanometer range, far beyond the resolving power of the human eye.
Q2. Why does a gas fill its container completely?
Gases have negligible intermolecular forces, allowing their particles to move freely and randomly in all directions. This results in the gas spreading out to occupy the entire volume of its container.
Q3. Convert 300 K to Celsius.
°C = K − 273
300 − 273 = 27 °C
300 − 273 = 27 °C
Q4. What is the melting point of ice in Kelvin?
The melting point of ice is 273 K (which equals 0°C).
Q5. What is the boiling point of water in Kelvin?
The boiling point of water is 373 K (which equals 100°C).
Q6. What is the physical state of water at 0°C?
At 0°C, water exists as a mixture of solid (ice) and liquid (water), since it is the melting point.
Q7.Arrange the following in increasing order of force of attraction between particles: Oxygen, sugar, water.
Oxygen < water < sugar, Gases have the weakest intermolecular forces, then liquids, then solids (sugar).
Q8.We can smell perfume from several meters away. Explain why.
Perfume molecules diffuse through air. The perfume particles spread out into air (fill the space) and reach our nose, so we smell them at a distance
Q9.A diver cuts through water in a pool. Which property of matter does this demonstrate?
This shows that water’s particles have space between them. The diver moves between molecules, indicating water molecules are not tightly locked.
Q10.Define matter. Give an example.
Matter is anything that occupies space and has mass.
Example: water (it fills a volume and has mass).
Example: water (it fills a volume and has mass).
📄 Short Answer Questions (2 Marks)
Q1. Why are gases more compressible than solids and liquids?
Gases have large spaces between their particles and very weak intermolecular forces. When pressure is applied, these particles can be pushed closer together. In solids and liquids, the particles are already tightly packed, offering very little room for compression.
Q2. What is evaporation? List two factors that affect it.
Evaporation is the process by which a liquid changes into vapour at temperatures below its boiling point. It occurs at the surface and leads to cooling. Two factors affecting evaporation are:
- Temperature – Higher temperature increases the rate of evaporation.
- Surface Area – A larger surface area allows more particles to escape quickly.
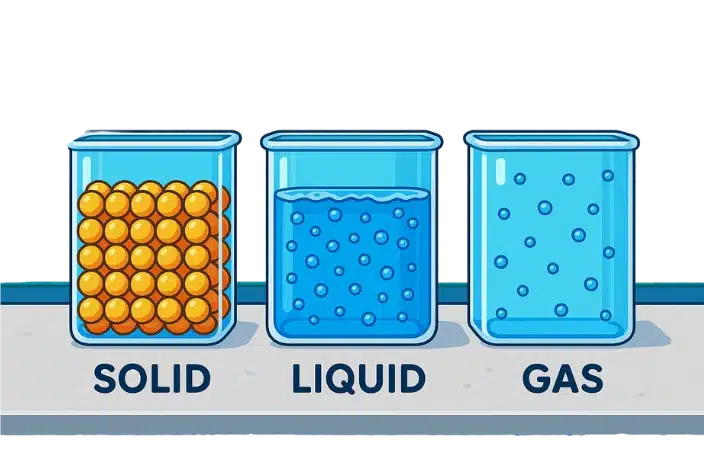
Q3. Compare solids, liquids and gases on the basis of shape, volume and compressibility.
| Property | Solids | Liquids | Gases |
|---|---|---|---|
| Shape | Definite | No fixed shape | No fixed shape |
| Volume | Definite | Definite | No fixed volume |
| Compressibility | Negligible | Slight | High |
Q4. Why do we feel cool when sweat evaporates from our skin?
Sweat evaporates by absorbing heat energy from the skin. This heat is used to break intermolecular bonds in the sweat. As a result, our body loses heat, which produces a cooling effect.
Q5. State four properties of liquids.
- Liquids have a definite volume (they occupy a fixed amount of space).
- They flow and take the shape of their container.
- They are almost incompressible (very low compressibility).
- Liquids have weaker intermolecular forces than solids but stronger than gases.
Q6. Why are solids almost incompressible?
In solids, particles are tightly packed with very little space between them. There is no extra space for particles to move closer under pressure. Hence, solids cannot be easily compressed (their volume hardly changes).
Q7. Give one difference between a gas and a vapor.
A gas is a substance in its gaseous state at room temperature (e.g. O₂, H₂) and is stable. A vapor is gaseous form of a liquid/solid below its boiling/melting point; it is unstable and condenses on cooling (e.g. water vapor).
Q8. Give reasons:
(i) A gas fills its container completely.
(ii) A gas exerts pressure on the walls.
(i) A gas fills its container completely.
(ii) A gas exerts pressure on the walls.
(i) Gas particles have negligible attraction and move freely in all directions; they spread out to occupy all available space.
(ii) Fast-moving gas particles collide with container walls; these collisions per unit area exert a force called pressure
(ii) Fast-moving gas particles collide with container walls; these collisions per unit area exert a force called pressure
📄 Short Answer Questions (3 Marks)
Q1. Explain why liquids have no fixed shape but a fixed volume. Why is the rate of diffusion in liquids higher than in solids?
Liquids flow and take the shape of their container because their particles are loosely packed and can move past each other. However, they have a definite volume because particles are still close enough that volume doesn’t change. Liquids diffuse faster than solids because their particles have more kinetic energy and more space to move. The greater kinetic energy in liquids (compared to solids) lets particles mix more quickly
Q2.Name the three states of matter. Which state has a definite shape, boundary and volume? Compare the three states in terms of compressibility.
The three states are solid, liquid, gas. Solids have a definite shape, distinct boundary and fixed volume.
Liquids take the container’s shape and fixed volume; gases fill the container and have no fixed volume.
In terms of compressibility:
Liquids take the container’s shape and fixed volume; gases fill the container and have no fixed volume.
In terms of compressibility:
- solids are essentially incompressible (negligible space between particles)
- liquids are slightly compressible (very small space between particles)
- gases are highly compressible (large spaces between particles allow volume to decrease greatly)
Q3. How does changing pressure affect the state of matter? Explain with a gas example.
Changing pressure can change the state of matter by forcing particles closer or allowing them apart. For gases, increasing pressure squeezes particles closer. If we also lower temperature, a gas can liquefy (e.g. turning natural gas into liquid under high pressure and cooling). Conversely, decreasing pressure allows gases to expand or even solids to sublimate.
For example, solid CO₂ (dry ice) at high pressure directly becomes gas when pressure drops to 1 atm
For example, solid CO₂ (dry ice) at high pressure directly becomes gas when pressure drops to 1 atm
Q4. Camphor or naphthalene balls in a wardrobe become smaller over time with no residue.
(a) Name the process.
(b) Is it physical or chemical change?
(a) Name the process.
(b) Is it physical or chemical change?
(a) This process is sublimation
– solid camphor/naphthalene turns directly into vapour without melting.
(b) It is a physical change , because the substance changes state (solid → gas) without forming a new substance.
(b) It is a physical change , because the substance changes state (solid → gas) without forming a new substance.
Q5. Give reasons:
(i) A gas completely fills the vessel it is in.
(ii) A gas exerts pressure on the walls of its container.
(iii) A wooden table should be classified as a solid.
(i) A gas completely fills the vessel it is in.
(ii) A gas exerts pressure on the walls of its container.
(iii) A wooden table should be classified as a solid.
(i) Gas particles move randomly and fill all available space because intermolecular attraction is negligible.
(ii) Rapidly moving gas particles collide with and push on container walls; these collisions produce pressure
(iii) A wooden table has a definite shape and fixed volume under normal conditions. Its particles are closely packed in a rigid structure, so it is a solid.
(ii) Rapidly moving gas particles collide with and push on container walls; these collisions produce pressure
(iii) A wooden table has a definite shape and fixed volume under normal conditions. Its particles are closely packed in a rigid structure, so it is a solid.
Q6. List three characteristics of the particulate nature of matter.
(i) The particles of matter have spaces between them.
(ii) The particles of matter are constantly moving.
(iii) The particles of matter attract one another.
(ii) The particles of matter are constantly moving.
(iii) The particles of matter attract one another.
📄 Long Answer Questions (4–5 Marks)
Q1. Compare in a table the properties of solids, liquids and gases (consider shape, volume, compressibility, diffusion, rigidity).
Matter exists in three main physical states: solids, liquids, and gases. Each has different particle arrangements and behaviors:
| State | Shape | Volume | Compressibility | Diffusion | Rigidity / Fluidity |
|---|---|---|---|---|---|
| Solid | Definite, fixed | Fixed, definite | Negligible (particles close) | Very slow (particles vibrate) | Rigid, maintains shape |
| Liquid | No fixed shape; takes container’s shape | Fixed, definite | Slight (particles close but can move) | Moderate (particles move freely) | Less rigid, flows easily |
| Gas | Fills container (no definite shape) | Fills container (no definite volume) | High (particles far apart) | Rapid (particles move fast) | Non-rigid, flows freely |
Q2. Explain the interconversion of states of matter with examples. What are the factors that affect these changes?
The phenomenon of change of matter from one state to another
and back to the original state is called interconversion of states of
matter.
Pressure: Brings particles closer; used to liquefy gases.
- Melting: The process of changing a solid into a liquid is called melting.The melting point is the temperature at which a solid changes into a liquid.
Example: When we heat an ice cube, it melts and turns into liquid water. The melting point of ice is 0°C. - Freezing: The process of changing a liquid into a solid is called freezing. The freezing point is the temperature at which a liquid changes into a solid.
Example:When we put liquid water in the freezer, it loses heat and freezes, forming ice. The freezing point of water is also 0°C. - Boiling:The process of changing a liquid into a gas at a specific
temperature throughout the liquid is called boiling. The temperature
at which a liquid boils is known as its boiling point.
Example: When we heat water in a kettle, it reaches its boiling point and starts boiling, forming steam. The boiling point of water is 100°C. - Condensation: The process of changing a gas into a liquid is called
condensation. It occurs when a gas loses heat energy and turns into
a liquid.
Example: When steam comes in contact with a cold surface, such as a mirror, it condenses and forms water droplets. This phenomenon is commonly observed in when you put a plate covering hot bowl if maggie, the vapours forms droplets. - Sublimation:It is the process in which a solid directly changes into
a gas without passing through the liquid state.
Example: Camphor(kapur), when heated, undergoes sublimation. It changes from a solid to a gas without forming a liquid in between. - Deposition: Deposition is the process that converts a gaseous state directly into a solid state without first going through a liquid phase.
Example:Frost is a common example of deposition because, without ever-changing into a liquid phase, water vapour from humid air are directly deposited on the solid and crystalline frost pattern on windows or on a leaf.
Pressure: Brings particles closer; used to liquefy gases.
Q3. What is latent heat of fusion and latent heat of vaporization? Explain why temperature remains constant during the melting or boiling of a substance.
Latent heat of fusion The amount of heat energy that is required tochange 1 kg of a solid into liquid at
atmospheric pressure at its melting point is
known as the latent heat of fusion.
Latent heat of vaporization The amount of heat energy that is required to change 1 kg of a liquid into gas at boiling point without any rise in temperature is known as the latent heat of fusion.
For example, ice melts at 0°C; heat absorbed breaks bonds and lets particles move freely, but temperature stays constant until all ice melts. Only after the phase change is complete does temperature rise again. Thus during melting/boiling, heat is “hidden” in changing the state, keeping temperature constant.
Latent heat of vaporization The amount of heat energy that is required to change 1 kg of a liquid into gas at boiling point without any rise in temperature is known as the latent heat of fusion.
For example, ice melts at 0°C; heat absorbed breaks bonds and lets particles move freely, but temperature stays constant until all ice melts. Only after the phase change is complete does temperature rise again. Thus during melting/boiling, heat is “hidden” in changing the state, keeping temperature constant.
Q4. Explain how evaporation causes cooling. Give two examples from daily life.
Evaporation is the vaporization of surface particles at temperatures below boiling. When particles evaporate, they absorb energy (latent heat) from the surroundings to overcome attractions. This energy absorption lowers the temperature of the remaining liquid and surroundings.
For example,
sweat on the skin evaporates and draws heat from the body, cooling us. Likewise, water sprinkled on a hot floor evaporates and removes heat, cooling the surface. Sitting under a fan increases air flow, which removes humid air and accelerates evaporation of sweat, enhancing the cooling effect
For example,
sweat on the skin evaporates and draws heat from the body, cooling us. Likewise, water sprinkled on a hot floor evaporates and removes heat, cooling the surface. Sitting under a fan increases air flow, which removes humid air and accelerates evaporation of sweat, enhancing the cooling effect
Q5. Explain why
(a) pouring acetone (nail polish remover) on your palm causes a cold sensation
(b) sitting under a fan in summer makes you feel cooler.
(a) pouring acetone (nail polish remover) on your palm causes a cold sensation
(b) sitting under a fan in summer makes you feel cooler.
(a) Acetone is volatile; its particles evaporate quickly at room temperature. As they evaporate from the skin, they absorb heat (latent heat of vaporization) from the palm, lowering its temperature. Thus the palm feels cold.
(b) Airflow from the fan increases the evaporation rate of sweat from our skin. Faster evaporation means more heat is drawn from the body into the sweat, causing a cooling effect. The moving air also carries away the humid air, maintaining a low humidity that favors further evaporation
(b) Airflow from the fan increases the evaporation rate of sweat from our skin. Faster evaporation means more heat is drawn from the body into the sweat, causing a cooling effect. The moving air also carries away the humid air, maintaining a low humidity that favors further evaporation
Q6. What is meant by the “diffusion” of gases? How is diffusion affected by temperature?
Diffusion is the mixing of particles of different substances by spontaneous motion, filling the available space.
Gaseous diffusion is very rapid because gas particles move randomly and quickly. Increasing temperature increases kinetic energy; particles move faster and spread out more quickly, so diffusion rate increases
Gaseous diffusion is very rapid because gas particles move randomly and quickly. Increasing temperature increases kinetic energy; particles move faster and spread out more quickly, so diffusion rate increases
📄 Case-Based Questions
Case Study 1 : Ria places a bowl of water under a fan and notices it gets cooler over time. Her brother spills perfume, and its scent spreads quickly across the room.
Q1: Why does water under the fan become cooler?
Answer: The moving air increases the rate of evaporation. As water evaporates, it absorbs heat from the remaining water and surroundings, making it cooler.
Q2: What property of gases allows perfume to spread across the room?
Answer: The rapid and random motion of gas particles (diffusion) allows perfume to spread quickly.
Q3: Which phenomenon is responsible for the cooling effect of evaporation?
Answer: Absorption of latent heat from the surroundings during evaporation.
Answer: The moving air increases the rate of evaporation. As water evaporates, it absorbs heat from the remaining water and surroundings, making it cooler.
Q2: What property of gases allows perfume to spread across the room?
Answer: The rapid and random motion of gas particles (diffusion) allows perfume to spread quickly.
Q3: Which phenomenon is responsible for the cooling effect of evaporation?
Answer: Absorption of latent heat from the surroundings during evaporation.
Case Study 2: Ravi observes camphor kept in a dish slowly disappears. He also notes that the floor becomes cooler after water is sprinkled.
Q1: What process causes camphor to disappear?
Answer: Sublimation – camphor changes directly from solid to gas.
Q2: Why does the floor become cooler after sprinkling water?
Answer: The water evaporates, absorbing heat from the floor, leading to a cooling effect.
Answer: Sublimation – camphor changes directly from solid to gas.
Q2: Why does the floor become cooler after sprinkling water?
Answer: The water evaporates, absorbing heat from the floor, leading to a cooling effect.
Case Study 3: Karan noticed that when he placed an ice cube on a metal plate and another on a wooden plank, the one on the metal melted faster. In another observation, he saw that petrol spilt on the floor evaporated quickly and gave a strong smell.
Q1: Why did the ice melt faster on the metal plate than on the wooden plank?
Answer: Metal is a good conductor of heat. It transfers more heat to the ice, making it melt faster. Wood is an insulator, so less heat reaches the ice.
Q2: Why does petrol give off a strong smell quickly?
Answer: Petrol is volatile. Its molecules evaporate quickly and mix with the air.
Q3: What property of particles does this demonstrate?
Answer: This demonstrates diffusion and that particles of matter are constantly moving.
Answer: Metal is a good conductor of heat. It transfers more heat to the ice, making it melt faster. Wood is an insulator, so less heat reaches the ice.
Q2: Why does petrol give off a strong smell quickly?
Answer: Petrol is volatile. Its molecules evaporate quickly and mix with the air.
Q3: What property of particles does this demonstrate?
Answer: This demonstrates diffusion and that particles of matter are constantly moving.
Case Study 4 : During an experiment, Arjun noticed that dry ice turned into a gas without forming a liquid. His teacher said it was used in fog machines.
Q1: What is the scientific name of dry ice?
Answer: Dry ice is solid carbon dioxide (CO₂).
Q2: What is the process observed by Arjun called?
Answer:The process is called sublimation.
Q3: Why is dry ice used in making artificial fog?
Answer: When dry ice sublimes, it produces thick white fog-like vapour used for stage effects and refrigeration without liquid residue.
Answer: Dry ice is solid carbon dioxide (CO₂).
Q2: What is the process observed by Arjun called?
Answer:The process is called sublimation.
Q3: Why is dry ice used in making artificial fog?
Answer: When dry ice sublimes, it produces thick white fog-like vapour used for stage effects and refrigeration without liquid residue.
Case Study 5 : A group of students measured the boiling point of water at a hill station and found it lower than 100°C. They were surprised and asked the teacher why this happened.
Q1: Why does water boil at a lower temperature at high altitude?
Answer: Atmospheric pressure is lower at high altitudes, so water needs less energy to boil.
Q2:How does atmospheric pressure affect boiling point?
Answer:Lower pressure means particles escape more easily, thus lowering boiling point.
Q3: What implication does this have for cooking at high altitudes?
Answer: It takes longer to cook food in boiling water, so pressure cookers are commonly used in such regions.
Answer: Atmospheric pressure is lower at high altitudes, so water needs less energy to boil.
Q2:How does atmospheric pressure affect boiling point?
Answer:Lower pressure means particles escape more easily, thus lowering boiling point.
Q3: What implication does this have for cooking at high altitudes?
Answer: It takes longer to cook food in boiling water, so pressure cookers are commonly used in such regions.