Is Matter Around Us Pure
Chapter 2 Summary – Is Matter Around Us Pure?
Ever wondered if the matter around us is truly pure? This chapter explores that curiosity by explaining the difference between pure substances and mixtures. It dives into homogeneous and heterogeneous mixtures, and unpacks the unique properties of solutions, colloids, and suspensions. With real-life examples and techniques like filtration, sublimation, and chromatography, you’ll learn how to separate mixtures effectively. The chapter also helps you identify physical vs. chemical changes in matter. Designed to simplify complex ideas, it sharpens your understanding of what makes a substance pure and why that matters in science — perfect for revision and exams alike.
NCERT Solutions for Class 9 Science Chapter 2 – Is Matter Around Us Pure? (2025 Edition)
📄 Page 15 – 📗 In-Text Questions
In other words, a pure substance consists of a single type of particle – for example, distilled water (H₂O molecules only), pure oxygen (O₂ molecules only), or crystalline sodium chloride (NaCl).
Unlike mixtures, a pure substance cannot be separated into other substances by physical means, because it already has only one constituent type
| Properties | Homogeneous Mixtures | Heterogeneous Mixtures |
|---|---|---|
| Uniformity of Composition | Homogeneous mixtures have the same composition throughout. | Heterogeneous mixtures do not have the same composition throughout. |
| Phases and Appearance | Homogeneous mixtures appear as a single phase (no visible boundaries between components). | Heterogeneous mixtures contain Two or more visible phases or regions. |
| Particle Size and Visibility | In homogeneous mixtures (solutions), particle size is extremely small (usually <1 nm) and cannot be seen. | Heterogeneous mixtures contain larger particles or chunks that can often be seen with the naked eye. |
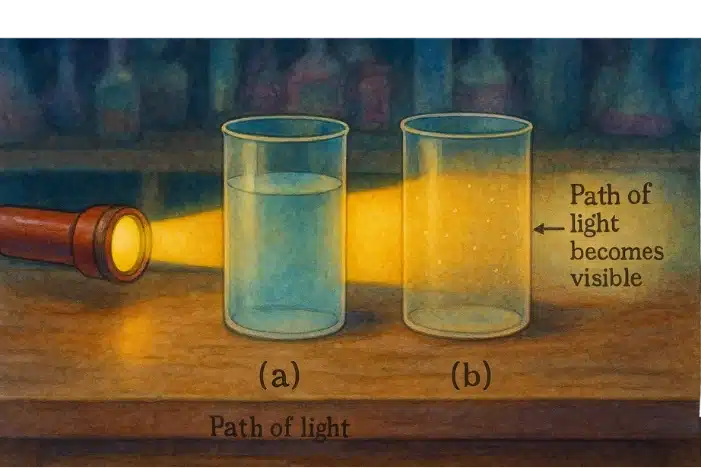
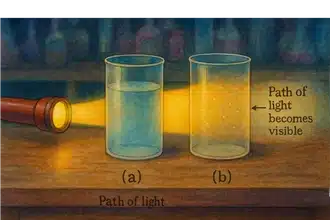
| Tyndall Effect | Homogeneous mixtures generally do not scatter light (no Tyndall effect). | Heterogeneous mixtures or colloids do scatter light. |
| Separation | Components of a homogeneous mixture cannot be separated by simple filtration. | Components of a heterogeneous mixture (like sand and water) can often be separated physically (e.g. by filtration). |
| Example | In a sugar-water solution the dissolved sugar is evenly distributed (homogeneous). | a mixture of iron filings and sand has visible distinct parts. |
📄 Page 18 – 📗 In-Text Questions
- Solution: A solution is a homogeneous mixture in which the solute (substance dissolved) is evenly distributed in the solvent (liquid, solid, or gas).
Solution particles are extremely small (< nm) and cannot be seen by eye or separated by filtration.
Solutions do not show the Tyndall effect (light beam passes through without scattering).
Example: Salt water or sugar water, where salt/sugar is the solute and water is the solvent. - Sol (Colloid): A sol is a type of colloid where tiny solid particles are dispersed in a liquid (solid-in-liquid colloid).
It is a heterogeneous mixture at the microscopic level.
The dispersed particles are small enough (larger than in a true solution but smaller than in a suspension) to remain suspended and can scatter light (Tyndall effect).
Example: Muddy water or paint; solid clay particles dispersed in water. Sols appear uniform to the naked eye but are actually heterogeneous mixtures. - Suspension: A suspension is a heterogeneous mixture in which relatively large solid particles are suspended in a liquid.
The particles do not dissolve and are visible to the naked eye.
Suspensions scatter light and are unstable: over time, particles settle down (e.g., muddy water settles to form a solid layer).
Example: A mixture of chalk powder in water or flour in water. Because the particles are large, they can be separated by filtration.
Mass of solution = mass of solute + mass of solvent = 36 g + 100 g = 136 g.
Using the mass-by-mass percentage formula:
Concentration (mass %) =
mass of solute
mass of solution
× 100 =
36
136
× 100 ≈ 26.5%
So the concentration is about 26.5% (w/w). (This uses the formula
mass of solute
mass of solution
× 100.)
📄 Page 19 – 📗 In-Text Questions
• cutting of trees,
• melting of butter in a pan,
• rusting of almirah,
• boiling of water to form steam,
• passing of electric current through water and the water breaking down into hydrogen and oxygen gases,
• dissolving common salt in water,
• making a fruit salad with raw fruits,
• burning of paper and wood.
Physical Changes
| Example | Description |
|---|---|
| Cutting trees | Only shape changes, no new substance formed |
| Melting butter in a pan | Change of state from solid to liquid |
| Boiling water to form steam | Liquid turns into gas, reversible change |
| Dissolving common salt in water | Salt is still salt, no chemical change |
| Making fruit salad with raw fruits | Just mixing pieces, no new substance formed |
Chemical Changes
| Example | Description |
|---|---|
| Rusting of an almirah | Iron reacts with oxygen to form rust (new substance) |
| Passing Of Electric Current Through Water and The Water Breaking Down into Hydrogen and Oxygen Gases | Water breaks into hydrogen and oxygen gases |
| Burning paper and wood | Combustion produces ash, CO₂, and other substances |
We note that physical changes can be reversed by physical means and the original substances remain (Group I in the iron–sulfur example involved physical mixing), whereas chemical changes produce different products (Group II’s heating reaction produced a compound).
Pure Substances
| Substance | Type | Description |
|---|---|---|
| Oxygen gas (O₂) | Element | Contains only oxygen molecules |
| Distilled water (H₂O) | Compound | Pure form of water, no impurities |
| Common salt (NaCl) | Compound | Has only sodium chloride particles |
| Sugar (C₁₂H₂₂O₁₁) | Compound | Single chemical compound |
| Pure copper metal | Element | Contains only copper atoms |
Each of these has only one type of chemical particle.
Mixtures
| Mixture | Type | Description |
|---|---|---|
| Air | Homogeneous | Mixture of nitrogen, oxygen, etc. |
| Sea water | Homogeneous | Water + various salts |
| Milk | Colloidal mixture | Water, fat, and proteins |
| Stainless steel | Alloy | Iron mixed with carbon and chromium |
| Soil | Heterogeneous | Rock particles and organic matter |
| Soft drinks | Homogeneous | Soda + sugar + flavorings |
These mixtures contain two or more substances physically combined.
According to NCERT, most naturally occurring materials (like minerals, soft drinks, soil) are mixtures of pure components, whereas pure substances have a fixed composition.
📄 Page 22 – 📘 Exercise Questions
| Mixture | Separation Technique | Reason / Explanation |
|---|---|---|
| (a) Sodium chloride from its solution in water | Evaporation / Distillation | Water evaporates, leaving NaCl behind; or use distillation to collect water separately. |
| (b) Ammonium chloride from a mixture of NaCl and NH₄Cl | Sublimation | NH₄Cl sublimes (directly turns to gas) and can be separated from NaCl. |
| (c) Small pieces of metal in engine oil | Filtration / Magnetic Separation | Filter out metal pieces or use a magnet if the metal is magnetic like iron. |
| (d) Different pigments from an extract of flower petals | Paper Chromatography | Pigments travel different distances on chromatography paper and separate. |
| (e) Butter from curd | Churning / Centrifugation | Butter separates out due to density difference during churning. |
| (f) Oil from water | Separation Funnel | Oil and water form separate layers due to difference in density; can be drained separately. |
| (g) Tea leaves from tea | Filtration | Tea leaves are retained on filter while tea passes through. |
| (h) Iron pins from sand | Magnetic Separation | Magnet attracts and removes iron pins from the mixture. |
| (i) Wheat grains from husk | Winnowing | Husk being lighter is blown away by air; heavier wheat grains fall straight. |
| (j) Fine mud particles suspended in water | Sedimentation & Decantation / Filtration | Mud settles at the bottom; water is poured off or filtered. |
📄 Page 23 – 📘 Exercise Questions
Answer: Making a cup of tea involves dissolving and filtering steps:
- Boil Water: Take water in a cup or pot and heat it. Water acts as the solvent.
- Add Sugar (Solute): Add some sugar. Stir and let it dissolve completely. Now you have a sugar-water solution, where sugar is the solute and is soluble in water. (Sugar becomes invisible as it dissolves.)
- Add Tea Leaves (Insoluble): Add tea leaves. Stir and boil briefly. The tea leaves do not dissolve (they are insoluble), but the colored compounds and flavor dissolve, coloring the water.
- Add Milk (Soluble): Add milk (optional). Milk mixes with the tea solution. Milk proteins and salts are soluble in water, making the tea lighter-colored.
- Filter: Pour the tea mixture through a strainer or filter into another cup. The liquid collected is the filtrate (the tea drink), and the tea leaves remaining on the strainer are the residue.
- What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 g of water at 313 K?
- Pragya makes a saturated solution of potassium chloride in water at 353 K and cools it to room temperature. What would she observe? Explain.
- Find the solubility of each salt at 293 K and identify the highest. (The solubility data from the table is: KNO3: 32 g, NaCl: 36 g, KCl: 35 g, NH4Cl: 37 g per 100 g water.)
- What is the effect of temperature change on the solubility of a salt?
- (a) From the solubility data at 313 K, 100 g water dissolves 62 g KNO3 to become saturated. For 50 g water, proportional scaling gives:
62 × (50 / 100) = 31 g
So 31 g of KNO3 will saturate 50 g of water at 313 K. - (b) On cooling the saturated KCl solution from 353 K to room temperature, KCl becomes less soluble. Excess KCl will precipitate out as solid crystals. Pragya would observe salt crystals forming (the solution becomes unsaturated on cooling) – this happens because solubility decreases with temperature.
- (c) At 293 K the solubilities (from the table) are:
- KNO3 = 32 g
- NaCl = 36 g
- KCl = 35 g
- NH4Cl = 37 g
- (d) Effect of temperature: Generally, the solubility of most solid salts in water increases with temperature. As temperature goes up, more solute can dissolve. (This explains why KNO3’s solubility jumps from 32 g at 293 K to 62 g at 313 K.) Conversely, cooling a saturated solution often causes some solute to come out of solution.
- Saturated solution
- Pure substance
- Colloid
- Suspension
-
Saturated solution:
A solution in which no more solute can dissolve at a given temperature. Beyond this point, any extra solute added remains undissolved.
Example: A solution with 36 g salt in 100 g water at room temperature – you cannot dissolve any more salt unless you heat or add more water. -
Pure substance:
A substance made of only one kind of particle. It can be an element or a compound, but has uniform properties throughout.
Examples: Table salt (NaCl), distilled water (H2O), oxygen gas (O2). These contain only one type of chemical molecule or atom. -
Colloid:
A colloid is a heterogeneous mixture in which the particle size of the dispersed phase is intermediate between that of a solution and a suspension, and it shows the Tyndall effect.
The particles (1–1000 nm) do not settle and cannot be filtered easily.
Examples: Milk (liquid fat droplets dispersed in water), blood, paint. These look uniform but scatter light due to suspended particles. -
Suspension:
A suspension is a heterogeneous mixture in which relatively large particles of a solid are dispersed in a liquid. The particles do not dissolve and are visible with the naked eye;
the mixture will scatter light and eventually settle.
Examples: Mud in water, chalk dust in water, or flour in water. If left undisturbed, the solid particles settle at the bottom.
Soda water, wood, air, soil, vinegar, filtered tea.
| Substance | Type of Mixture | Explanation |
|---|---|---|
| Soda water | Homogeneous | Solution of CO₂ gas uniformly dissolved in water; appears as a single phase. |
| Wood | Heterogeneous | Contains cellulose, fibers, and resins; components are visible and non-uniform. |
| Air | Homogeneous | Uniform mixture of gases like nitrogen, oxygen, etc.; appears as a single phase. |
| Soil | Heterogeneous | Mixture of minerals, organic matter, air, and water; visibly non-uniform. |
| Vinegar | Homogeneous | Solution of acetic acid in water; completely uniform with one visible phase. |
| Filtered tea | Homogeneous | Tea extract without leaves is uniform and appears as a single phase liquid. |
-
Boiling/Freezing Point: Pure water boils at 100 °C (373 K) and freezes at 0 °C (273 K) under normal pressure.
Measure the liquid’s boiling or freezing point – if it matches these values precisely, it is likely pure water.
Note: Any dissolved impurities usually raise or lower these points (boiling point elevation or freezing point depression). - Electrical Conductivity: Pure water is a very poor conductor of electricity (contains no free ions). If the liquid conducts electricity strongly, it indicates the presence of dissolved ions (impurities).
- Chemical Test: Add a small amount of anhydrous copper sulfate (white powder). If it turns blue, the liquid is water, as it forms CuSO4·5H2O. Non-water liquids usually do not cause this change.
- Tyndall Effect: Shine a narrow beam of light through the liquid. Pure water does not scatter light and shows no beam. If light scatters visibly, it may be a colloid or impure.
Note: Pure substances like water have fixed and characteristic physical and chemical properties.
| Material | Type |
|---|---|
| Ice | Pure Substance |
| Milk | Mixture |
| Iron | Pure Substance |
| Hydrochloric acid | Pure Substance |
| Calcium oxide | Pure Substance |
| Mercury | Pure Substance |
| Brick | Mixture |
| Wood | Mixture |
| Air | Mixture |
| Mixture | Type |
|---|---|
| Soil | Not a Solution |
| Sea water | Solution |
| Air | Solution |
| Coal | Not a Solution |
| Soda water | Solution |
| Substance | Tyndall Effect |
|---|---|
| Salt solution | No |
| Milk | Yes |
| Copper sulphate solution | No |
| Starch solution | Yes |
| Substance | Classification |
|---|---|
| Sodium | Element |
| Soil | Mixture |
| Sugar solution | Mixture |
| Silver | Element |
| Calcium carbonate | Compound |
| Tin | Element |
| Silicon | Element |
| Coal | Mixture |
| Air | Mixture |
| Soap | Compound |
| Methane | Compound |
| Carbon dioxide | Compound |
| Blood | Mixture |
| Process | Type of Change |
|---|---|
| Growth of a plant | Chemical Change |
| Rusting of iron | Chemical Change |
| Mixing of iron filings and sand | Physical Change |
| Cooking of food | Chemical Change |
| Digestion of food | Chemical Change |
| Freezing of water | Physical Change |
| Burning of a candle | Chemical Change |